Unwinding the situation behind a presumed conservation success.
Note: this post will be updated as further information becomes available.
Simandoa conserfariam, known as the “Extinct in the Wild Roach,” “Extinct Roach,” “Simandoa Cave Roach” and the “Conservation Roach,” is a roach species from West Africa that has spread across the globe in captive collections. Easy to care for, prolific, and pleasingly colored, its popularity with roach hobbyists belies a darker fate in the wild.

S. conserfariam was discovered in 2002 in a cave in the Pic de Fon Classified Forest, an ecologically-diverse area situated on the slopes of the Simandou Mountains of south-eastern Guinea. The remote mountain range is famous for its biodiversity, one of 36 global biodiversity hotspots recognized by the Critical Ecosystem Partnership Fund. It is home not just to S. conserfariam but also to more charismatic megafauna, including threatened chimpanzee populations and a diverse cast of bats.
Look below the surface however, and the Simandou Range reveals that it is not only one of the richest regions ecologically, but also one of the richest mineralogically. In the late 1990s Rio Tinto, the second largest mining company in the world, began surveying the area for iron ore. Later, in 2008, the Guinean government estimated that the Simandou Range held 8.6 billion metric tons of iron ore, making it the largest untapped source of high-grade ore in the world.
The sheer monetary value of such a discovery led to massive legal battles over the mining rights in the Simandou Range that drew in not only Rio Tinto and the Guinean government, but also companies like Vale SA, Beny Steinmetz Group Resources, and the Chinese state-owned Chinalco. Though the ore deposits have been known about for decades, legal wrangling has prevented it from ever being extracted, though construction for eventual large-scale mining has continued, including in the Pic de Fon Classified Forest (PFCF).
The PFCF, located in the southern end of the range, is one of a few protected regions in the Simandou Range, established by the French colonial government in 1953 as a conservation area and preserved by the Guinean government after Guinea’s independence in 1958. Though it has been legally protected for the entirety of its 71-year existence, due to its remoteness, regional political instability, and governmental corruption its biodiversity has never been adequately studied, much less protected.

There are no known current conservation initiatives for the PFCF, and the only available management plan for the forest, published in 2010 by the Guinean government and the forestry organization Centre Forestier de N’Zérékoréin, allows Rio Tinto, at the time the company with control of mining rights to the range, to manage conservation operations in the area. It provides no specifics for protecting the forest’s ecology or limitations on what Rio Tinto can do within the PFCF. Furthermore, there is no information available online about the forest’s present protections.
Thus, though large-scale exploitation of the Simandou Range’s ore deposits has faltered, construction for roads and railways to eventually move iron ore to ports Guinea’s coast has continued in the PFCF under both Rio Tinto and various Chinese mining companies, while logging, slash-and-burn farming practices, and cattle grazing from local farmers and communites have eaten away at the PFCF’s borders. The lack of protection has allowed anyone with the right connections in the Guinean government free access to the area.
Though what information that is available about the Simandou Range’s biodiversity shows that it is indeed a global hot spot for species richness, the remoteness, political difficulties, and legal challenges have made it difficult for any extended study of it. In fact, the mountains’ were so understudied that it was difficult for Rio Tinto, the first company to try and exploit the mountains’ iron ore in the late 1990s and early 2000s, to comply with international conservation law. In order to continue its operations in the area, in 2002, Rio Tinto called in Conservation International (CI).
CI, a conservation organization formed in 1987, decided to perform a Rapid Assessment Program (RAP) of the range. Since 1990, CI had conducted RAPs in understudied regions around the world. Comprised of small, flexible teams of conservation scientists that rapidly catalogued a region’s biodiversity, RAPs were “ecological SWAT teams,” according to CI’s founder, Peter Seligmann. With scientists drawn from all branches of the life sciences, RAP teams were capable of finding and collecting vast numbers of species during several weeks spent in the field. Later, back home in labs around the world, RAP members worked to identify and, in many cases, describe their finds as quickly as possible in order to help the scientific community and companies like Rio Tinto understand what lived in the region and how to best protect it.
The RAP team sent to the Simandou Range in 2002 included 13 biologists of varying specialties, and examined two different sites in the range in November-December of that year, including a location in the PFCF. Overall, the team cataloged at least 797 species, including several new species of plants and katydids, a frog, and a small, unusual roach.

While many roaches choose to live above ground in leaf litter or tree trunks, S. conserfariam was found in a bat cave. Piotr Naskrecki, one of S. conserfariam’s discoverers, recounted in the paper describing the species that the cave was “…open on both ends, allowing for a considerable amount of light to penetrate most of its area. The distance between the openings was about 35 m, and the distance from the floor of the cave to the highest point of its ceiling was about 20 m…humidity was high, maintained by a small stream running through it.”
Guano from thousands of Egyptian fruit bats (Rousettus aegyptiacus) covered the cave floor, in some areas to a depth of a meter/three feet. In this guano, colorful roaches were found by the thousands, usually in large aggregations of several dozen individuals. Two other species of roach, a tenebrionid beetle species, and Phaeophilacris cave crickets were also found in the guano and on the cave walls. Naskrecki and Louis M. Roth, another entomologist, collected several of these roaches for study, and a year later, in 2003, it was given a name: Simandoa conserfariam.
While the RAP expedition was successful in documenting the PFCF’s biodiversity, it was less so in protecting the forest, and the Simandou Range more broadly, from mining development. What Rio Tinto did with any recommendations provided by the RAP expedition is unclear, and development of the area has continued apace despite numerous setbacks. This year The Assay reported that Rio Tinto recently approved funding for a massive new development in the region that will hopefully start producing iron ore by 2025.
However, as early as 2013 Naskrecki and others were warning of the possible extinction of S. conserfariam. Naskrecki wrote on his blog that “The cave was on the path of a major road that was being constructed and it may no longer exist.” Satellite imagery obtained through Google Earth shows an inconclusive picture, but no defined cave entrances can be seen. Imagery from before the construction for comparison has not been attained. Further development of the area for mining seems highly likely.

Since the cave’s original discovery in 2002, the only other known expedition to it was during a 2008 bat survey. Led by mammalogist Jan Decher, it found only one Egyptian fruit bat in the cave. Reasons why the population might have been so reduced, and the state of the cave, were not discussed in the paper describing the results of the expedition, but Decher later clarified over email that the cave, called “Whiskey 2,” was not damaged when he visited.
Decher further noted that the bats probably only occupy the cave seasonally, though disturbances from the nearby road could have driven them away. He did not see any roaches, but at the time he was unaware of the description of S. conserfariam and did not look for them. Decher also cautioned that while there may be other caves in the area, many of the cave-dependent bat species do not necessarily need caves like the one S. conserfariam was found in. They could instead roost in rock piles, crevices, or overhangs.
While the cave has remained unvisited since 2008, it is possible the stream that ran through it has been visited as recently as 2022. From 2011 to 2012, a team of researchers led by Oi Edia Edia surveyed the aquatic insects of streams around the Simandou Range, publishing their findings in 2016. One of the streams they sampled was called “Whisky 2,” and was found to have a disturbance factor, or the degree to which it had been damaged, of “None;” other, nearby streams were also undisturbed.
While, the GPS coordinates provided for “Whisky 2” do not match those of the cave provided by Naskrecki and Roth in their paper describing S. conserfariam, instead pointing to a spot farther down the western slopes of the mountains, this could be because the stream was sampled at a lower elevation, or because it joined another, larger stream somewhere below the cave. Though its endpoint is not known for sure, if “Whisky 2” is the same stream as the one that runs through the “Whiskey 2” cave mentioned in both Naskrecki and Roth’s description and in Decher’s paper, it is unlikely that the cave had been destroyed at the time. If it had been, the stream would show signs of fouling and damage as it eroded a new path for itself out of the cave.
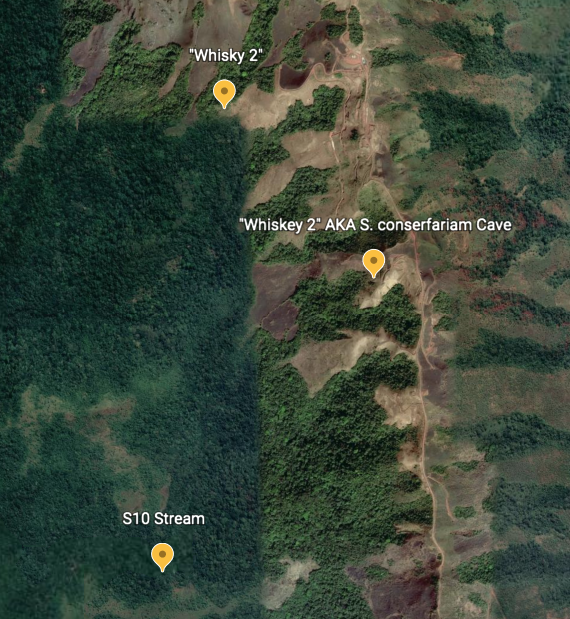
Over email, Edia, said that he did not know if the stream was the same, though he had worked on the “Whisky 2” stream in 2022. He further noted that mining in the region was stopped in 2014-2015, and had only recently started again farther north near Oueleba Mountain. Because of this, Edia said that “I am more than reassured that this cave still exists.”
Whether or not the cave is gone, S. conserfariam is still very much alive. Naskrecki, along with the preserved specimens used for his description, collected living specimens: one male, three females, and several nymphs. He brought them to Harvard University to establish a colony, and almost ten years later, in early 2012, he sent specimens to Peter Clausen of Bugs In Cyberspace, one of the largest pet invertebrate retailers in the United States.
Clausen established his own colony and later sold an estimated thousands of specimens to other roach enthusiasts in the US. The species then spread to collectors in Europe and Asia, thus fulfilling its name conserfariam, which means “conserve in many places.” Even so, there is no official recognition of S. conserfariam as extinct in the wild by the International Union for Conservation of Nature (IUCN), an organization that tracks extinctions through its Red List of Threatened Species.
Of course, S. conserfariam might simply have been missed amidst broader conservation priorities; Lucihormetica luckae, an Ecuadorian roach that is widely believed to be extinct, is similarly not on the IUCN Red List. This discrepancy between S. conserfariam‘s reputation as extinct in the wild and its official recognition of such might also be due to the limited information available on its range in the wild. Roth and Naskrecki wrote that it was unknown if the roaches could be found outside the cave, and given S. conserfariam‘s easy adaptation to a wide range of captive conditions, it seems quite possible that the roach could be found outside in the forest. The cave might have been a place where the roaches could proliferate easily, but not be their only or even their primary habitat.
Even if caves were S. conserfariam‘s primary habitat, the specific cave they were found in was only one of many in the Simandou Range. The 2002 RAP also surveyed bats, finding at least 10 species dependent on caves as resting places. The scientists studying the bats, Jakob Fahr and Njikoha M. Ebigbo, noted that “The bat fauna [of the Simandou Range] is also characterized by the high proportion of species that depend strictly…or partially…on caves.” However, they also pointed out that only one bat cave was studied during the RAP expedition, and that was the cave with the Egyptian fruit bats and S. conserfariam. Might other caves where S. conserfariam lives be out there?
Regardless of whether or not the extinct roach’s cave still exists, or whether its primary habitat is even caves at all, the species has not been documented in the wild since 2002. Thus the question remains: does the extinct roach still exist in the wild, or is it truly gone from its natural habitat? And while it is most certainly not completely extinct, its fate raises questions about the rest of the Simandou Range’s biodiversity that is unknown and potentially threatened. How many species were lost without ever being known, if any?
Over 20 years later, the case of S. conserfariam still raises more questions than it answers.




























































 I counted five or six ant species in the backyard alone, including this photogenic little Camponotus sp. Identified by ponerinecat.
I counted five or six ant species in the backyard alone, including this photogenic little Camponotus sp. Identified by ponerinecat.





















































































