Author: Arthroverts
-
Cockroaches & Comics – An Interview with Maddy Ruth
Earlier this year, I received an email inquiring about my story on Simandoa conserfariam, the extinct in the wild roach. The sender was Maddy Ruth, a multimedia artist working on a comic book. We exchanged a few emails about my sources and whether or not the cave S. conserfariam was found in still existed, and…
Written by

-
Scorpions, Shrews, Nudibranchs, Oh My! – An Interview with Prakrit Jain
“Get out in the field if you can, and if not, try to contact others who have to learn from their observations.”
Written by

-
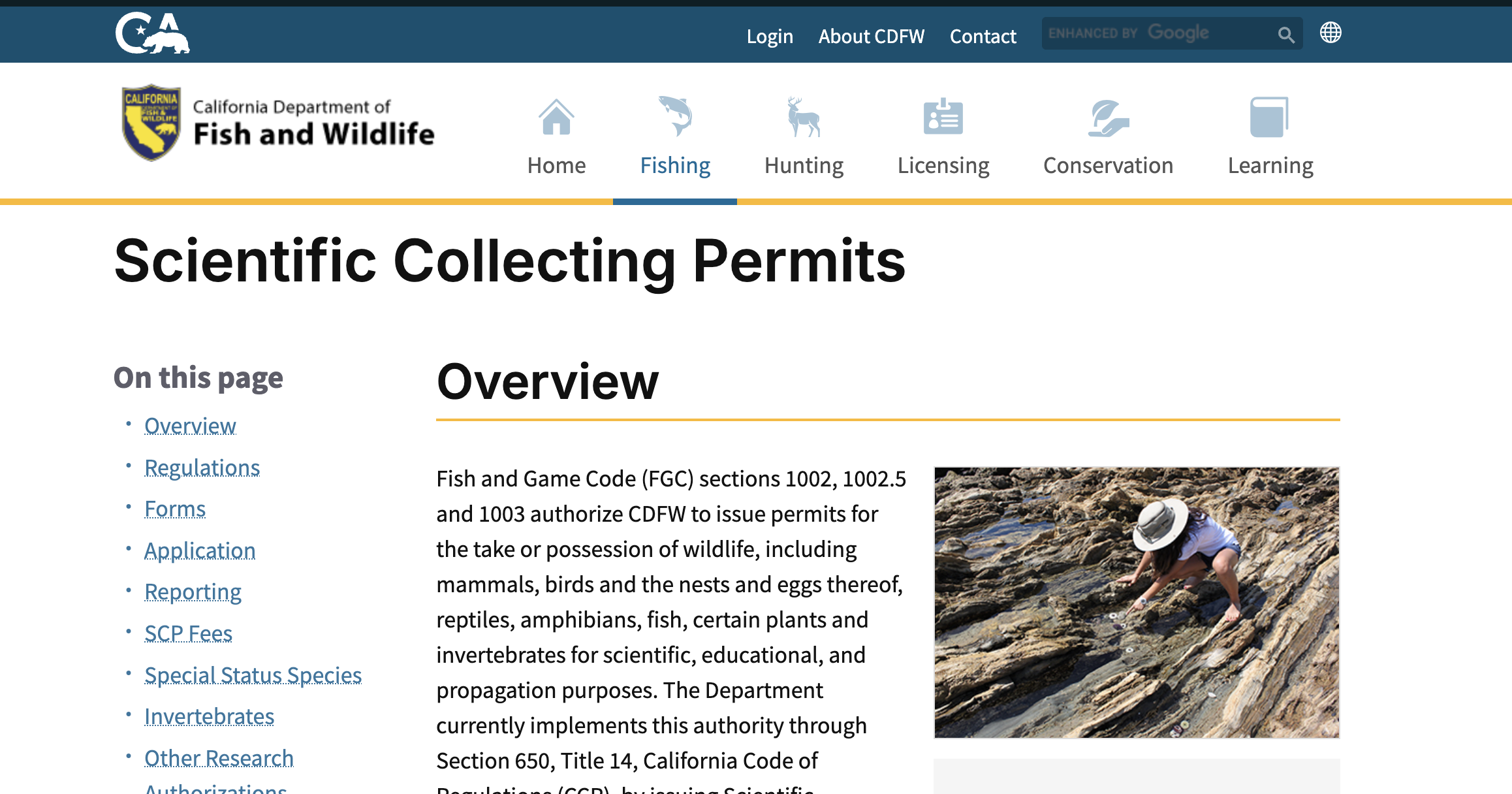
Report from Town Hall on CDFW Scientific Collecting Permits
Biodiversity scientists from across California gathered to field suggestions for CDFW’s Scientific Collecting Permit application process.
Written by
-
In Search of Isopods – An Interview with Oonagh Degenhardt (aniedes)
“The most rewarding part for me has to be finding a species exactly where I expected it to be…”
Written by

-
The ongoing mystery of the Extinct in the Wild Roach
Easy to care for, prolific, and pleasingly colored, the Extinct in the Wild Roach’s popularity with hobbyists belies a darker fate in the wild. But is it actually gone?
Written by

-
An Interview with Dr. Derek Hennen!
“I found it at night using a UV flashlight and it glowed a beautiful blue-green color, it was just astounding. It blew my mind and I decided ‘okay, this is what I want to study.'”
Written by

-
A Living Amber Scorpion
…I did discover one other reason why Arizona continues to draw invertebrate enthusiasts from around the world: the scorpions.
Written by

-
Velvet Worms in Need of Some Help…
…When he first discovered the species in his yard in 1990, he found a colony of over 2,000 specimens living under a brick pile!
Written by

-
Migidae sp. “Madagascar” and Conserving These Beautiful Spiders
…Dealing with something so old has made me completely change how I view keeping, and by extension conserving, trapdoor spiders.
Written by

